Is Decanting a Chemical or Physical Change
Examples of chemical changes are burning cooking rusting and rotting. We can obtain the substance back even after the change.

Decantation Mixtures Definition Examples Applications
No new substance is formed.
. The solid table salt is added to the water and disappears. Chemical changes usually involve the production of energy which can be in the form of heat light sound etc In a physical change no new substance is formed. Distillation is an example of physical change.
What is the difference between chemical and physical changes. Most of the physical changes are reversible. In its simplest form it just means allowing a mixture of solid and liquid or two immiscible liquids to settle and separate by gravity.
It is made up of tiny particles and has physical and chemical properties. When matter undergoes a chemical change it cant return to its original state without additional reactions. Gas to solid deposition.
Some characteristics of a physical change are. Evaporation does not result in any chemical reaction nor any breaking or making of chemical bonds. Generally physical changes do not involve the production of energy.
Up to 24 cash back Physical and Chemical process. No chemical bonds are disrupted in this process except. Physical vs chemical changes 92215.
Does not affect the internal structure of a substance only the molecules are rearranged. The dissolution of an ionic solid in water is manifestly a chemical change in that strong electrostatic bonds have been broken and new bonds generated in the new substances that have been formed. Sometimes it is difficult to determine whether a change is physical or chemical.
PhysicalChemical process The Physical Process. Note the properties of a mixture may be different from its components. This dissolution is easily identified as a physical change because if the water is.
Decanting is also a chemical laboratory process used to separate mixtures. Decanting is also a chemical laboratory process used to separate mixtures. Chemical change a process in which chemical bonds are broken or created to make a new substance.
The melting of a candle is a physical change while the burning of a candle is a chemical change The freezing of any liquid matter as water is a physical change. It is not a change physical or chemical. Solid to liquid fusion.
These filters clean water through physical processes. Learn vocabulary terms and more with flashcards games and other study tools. Physical properties of matter include its appearance and observable properties.
They perform the same. Start studying Chemistry. In contrast a chemical change is one in which substances change into different substances with different formulas.
And decomposition involves the breaking and making of chemical bonds and the formation of new substances. A chemical change is one in which changes to chemical. This is a physical change because its not permanent and no chemical reaction occurs.
To manually decant all of a liquid. A few examples of chemical change are digestion of food burning of coal rusting etc. For example think about dissolving table salt sodium chloride into liquid water.
Chemical changes MAY take place after some liquids are removed from their container and exposed to air but decanting by itself is not a. And thus decomposition should fall. Liquid to gas vaporization.
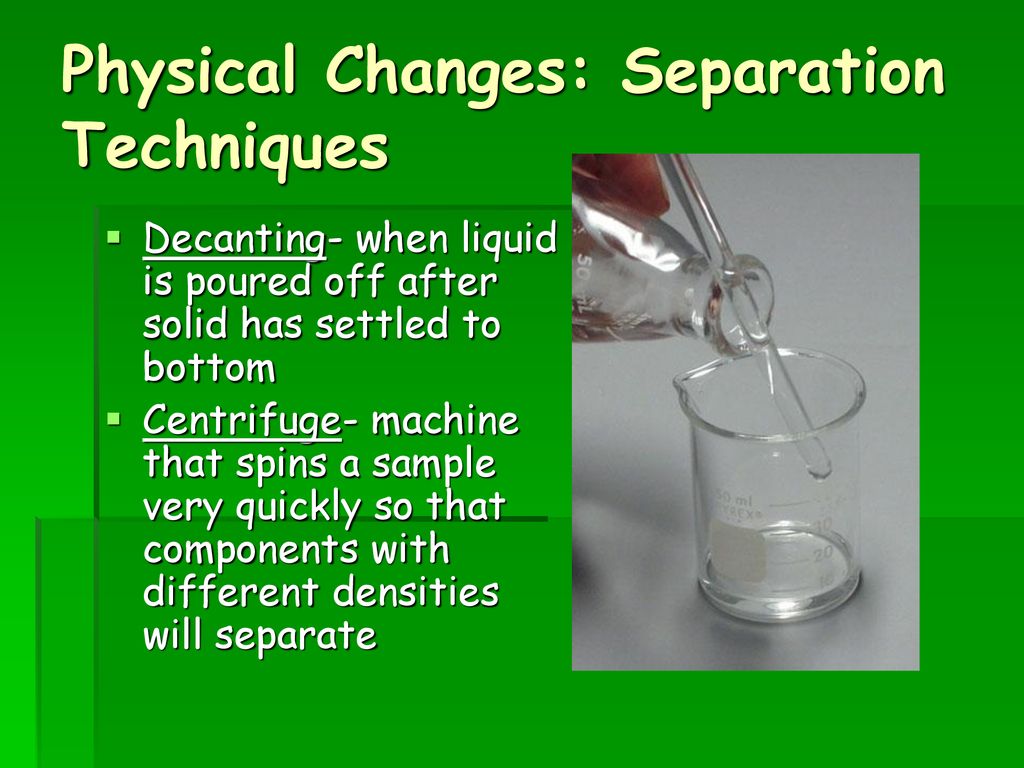
Decanting is done to separate particulates from a liquid by allowing the solids to settle to the bottom of the mixture and pouring off the particle-free part of the liquid. Changes can be either physical or chemical. Chemical Change or Physical Change.
Many physical changes are reversible if sufficient energy is supplied. Physical changes are often changes of state. Decantation is a process for the separation of mixtures by removing a layer of liquid generally one from which a precipitate has settled.
Decanting is a process used to separate mixtures and ii its simplest form it just means allowing a mixture of solid and liquid or two immiscible liquids to settle and separate by gravity. The only way to reverse a chemical change is via another chemical reaction. Melting and dissolving are examples of physical changes.
In a physical change the substance retains its identity no chemical reactions are involved. Examples of physical changes are boiling melting freezing and shredding. The purpose may be either to produce a clean decant or to remove undesired liquid from the precipitate or other layers.
Physical change a process in which a substance changes its state of matter but chemical bonds stay intact. The evaporation of the water to form the water vapour and the condensation of the water vapour into the water drops are physical changes The boiling of water and the melting of any solid matter. Decantation is sometimes mistakenly used interchangeably with the word filtration.
Its chemical composition remains intact. In everyday life the term decantation is usually associated with wine. Updated on November 25 2019.
This type of water filter is known as a multimedia filter. Some physical properties are colour odour taste solubility rigidity. Mixtures - Mixing together materials where one is not soluble in the other is a physical change.
If you consider water before evaporation it is H_2Ol if you then consider water after evaporation it is H_2OgIt is still H_2O - the molecule hasnt changed all that has changed is the physical state. A physical change or process is usually one that involves a change of state. On the other hand the dissolution of say ethyl alcohol in water or petroleum ether is probably an example of physical change in that no new substances have.
Examples of Decantation For example a mixture possibly from a precipitation reaction is allowed to stand so that gravity has time to pull the solid to the bottom of a container. The term matter refers to anything that has mass and occupies space.

0 Response to "Is Decanting a Chemical or Physical Change"
Post a Comment